How do I handle mice?
Most strains of mice are quite docile, and will not bite unless provoked. If the mice become upset, do something else and return to them after they have calmed down.
Transferring mice from cage to cage. Gently pick up the mouse by the tail with a gloved hand. Grasping the mouse by the base of the tail will give you greater control. Tailless mice may be grasped by the scruff of the neck. Relax and handle the mice gently. In most circumstances, if you are relaxed, if the mouse makes an attempt to bite you, it will be a gentle exploratory nibble that will not hurt. A few strains, particularly wild-derived strains, are very active and can pose problems for handling. Long forceps (Fisher 10-316C) are helpful with these strains. Forceps should not be used unless necessary for the protection of the handler.
Checking plugs, inspecting mice. For checking plugs, and some other observations, a little more control of the mouse is required. Transfer the mouse to the top of the cage with the bars of the cage running left-right. Grasp the mouse by the base of the tail between thumb and forefinger and place the rest of your fingers of the grasping hand on the sacrum and lumbar region of the mouse. When you grasp the mouse, it will pull away from you with its front legs on the bar of the cage, enabling you to inspect its nether regions.
Handling mice for marking, injections and gavage. Complete control of the mouse is required for marking, injections and gavage. The following instructions will result in a mouse immobilized in your left hand, with the right hand free. Place the mouse on top of the cage bars, with the bars running left-right. Grasp the tail with your right hand and then scruff the mouse with your left hand. Scruffing the mouse properly is the key to success. You want to immobilize the head of the mouse, and to accomplish this, all loose skin around the back of the neck must be scruffed. To get all this skin in your grip, start your scruffing motion low on the shoulders of the mouse, thumb on one side, and index finger on the other. The skin on the back of the mouse can be trapped between the remaining fingers and palm. For gavage and injection, the body of the mouse needs to be immobilized as well: stretch the body of the mouse by gently pulling the tail with your right hand and hook the little finger of your left hand over the tail. A more extensive, detailed description of how to perform gavage is given here.
If you are not constrained to work on the mice at a particular time of day, you may find it easier to work on the mice in the morning to early afternoon. Mice are at their most active just before the lights go out, and are often more difficult to handle late in the day.
How do I tell males from females?
The distance between the external genitalia and the anus is greater in males than in females at all postnatal stages. After about two weeks of age, the nipples of females are typically visible, whereas the nipples of males are not. In adults, the scrotum of the male (and testes, if they are everted) is an obvious marker. For pre-adults, orient the cage so that the bars run left-right and put the mouse on the wire rack of the cage. Grasp the tail of the mouse between the thumb and forefinger, and place the other fingers on the back of the mouse and bend the butt end of the mouse up to you. The mouse will try to pull away from you using the bars of the cage. It is easiest to sex newborn mice if the genital region of the mouse is fully extended: pick the mice up and gently bend the lower back slightly to stretch the genital region. In pigmented strains, male newborn mice have a spot of pigment over the scrotum. At about two weeks of age, the nipples of female mice are more prominent than those of males. Embryos and fetuses can be typed by PCR with the primers SMCX-1 5'CCGCTGCCAAATTCTTTGG3' and SMC4-1 5'TGAAGCTTTTGGCTTTGAG3'. Females give a single band and males give two bands because of an intron difference between the X and Y genes (Agulnik et al. 1997 Mamm. Genome 8, 134-138.) Alternatively, Jarid 1c F CTGAAGCTTTTGGCTTTGAG & Jarid 1 c R CCACTGCCAAATTCTTTGG primers amplify a band of 331 bp in females but two bands of 302 and 331 bp in males: Clapcote SJ, and Roder JC. Biotechniques 2005 38(5): 702. Simplex PCR assay for sex determination in mice. PMID: 15945368.
How do I mate mice?
If you are not in a rush to produce a lot of offspring, house a male mouse with one or two female mice. The mice can be left together until the pups are ready to be weaned if the cage doesn't get too crowded. If you need mice of predominantly of one sex, you can remove the unwanted sex a few days after birth (don't disturb the moms during the first 24 hours after birth). The remaining pups will grow faster. However, you should be aware that females are better mothers if they have at least 3 pups to care for, so don't cull too severely. If you need to expand a strain quickly, you can mate females in estrus with the males every day and check plugs the next morning. House females of similar plug dates together through to weaning of the pups. For many strains, two pregnant females and their litters can be housed together until weaning, although you may find that particularly fecund strains like CD1 require that the cage be split to avoid overcrowding. The IACUC guidelines for mice with litters limit the number of mice to 2 adults and no more than 20 pups.
How long is gestation?
Gestation is 18 to 20 days, depending on the strain.
How can I prevent mothers from cannibalizing their litters?
Mice are social and care better for their young when they are housed with friends. House females continuously with the sire, or house pregnant females together, or house a pregnant female with a non-pregnant female. However, do not add mice to a cage just a few days before birth, as this will disturb them. First time mothers and very young females are less likely to raise a litter successfully than experienced mothers and more mature females. Mothers and their litters should not be disturbed the first day after birth. By the second day, mothers should have acquired full maternal behavior and will tolerate disruptions better. Adverse environmental conditions such as sudden loud noises and inadequate ventilation can also have a detrimental effect. Some strains are more maternal than others (see Jax' listing of strain characteristics). In difficult situations, you can foster the pups to a more maternal strain or cohouse a pregnant mouse of a maternal strain at the same or more advanced stage of pregnancy (with a different coat color) together with your problematic mom. You can keep one or several cages of outbred mating pairs (for example, CD1 mice from Charles River) on hand for fostering pups. A detailed description of how to foster mice provided by the Jackson Laboratory is available here. In addition, see the section below on increasing reproductive performance.
When should mice be weaned?
Mice should be weaned at 3 to 4 weeks after birth. Pups must be weaned if the same mom gives birth to a second litter. The pups should be robust, active, have open eyes, teeth and adult fur rather than the sparser fur of babies. They need to be able to jump up to the top of the cage to feed and drink. If they are too immature, let them go longer with their mom. In many strains, pups ready to wean will "popcorn" when the cage lid is opened. If you are uncertain about their ability to well on their own, you can leave a little water-softened food in the bottom of the cage to help them through the first day or two.
When do mice become sexually mature?
Female mice become sexually mature at 6 weeks after birth and males at 8 weeks.
What are the acceptable methods of euthanasia?
Mice are narcotized by CO2 inhalation and then euthanized by cervical dislocation. Although CO2 alone can euthanize the animals, it must be ascertained if the animals have died (see the NIH guidelines on the use of CO2 alone), thus cervical dislocation is recommended after the use of CO2. CO2 must be delivered from a tank, not from dry ice. The ARC provides tanks and chambers. Let the gas flow for 1 minute to fill the chamber and leave the chamber closed for 5 minutes. The narcotization of mice is rapid, so do not leave the chamber unattended. Death should be ensured by cervical dislocation. In the different facilities at CWRU, mice can be left on the racks in a designated room to be euthanized by ARC staff. The mice must not be overcrowded, and must have sufficient food and water to last through working hours of the next working day. If unweaned pups are left without their mother, the ARC must be notified immediately so that euthanasia may be performed without delay.
CO2 for euthanasia is cheap, convenient, effective and poses little risk for staff and investigators, but the humaneness of its use is increasingly debated. An alternative method of euthanasia is to anesthetize the mice with isoflurane before cervical dislocation. In a chemical fume hood (i.e. an explosion-proof hood vented to the outside), put a cap-full of isoflurane on tissues in the bottom of a small chamber (an empty plastic pipet tip box for euthanizing single mice), place the mouse inside and close the chamber. When the mouse is immobile, open the chamber and perform cervical dislocation. Be aware that isoflurane is a health hazard, and exposure to personnel should be avoided by confining use to chemical fume hoods and anesthesia apparatus and by proper storage.
Mice may be euthanized by cervical dislocation without anesthesia by experienced, competent individuals, if scientifically justified. The IACUC may require demonstration of proficiency in cervical dislocation. Cervical dislocation is performed by picking up the mouse by the base of the tail. The mouse is allowed to grip the bars of a transversely oriented cage top and while pulling gently backwards by the tail, the base of the skull is firmly gripped between thumb and index finger. To ensure humane euthanasia, cervical dislocation should be learned under supervision of a qualified individual.
What are the acceptable methods of euthanasia for fetal and newborn mice?
Newborn mice can be narcotized in a small plastic bag with CO2 from a gas cylinder, the bag is sealed, then the mice are euthanized by placing in a freezer. Complete CWRU IACUC regulations and recommendations on acceptable methods of euthanasia of fetal mice (over 14 days of gestation), newborn mice and young mice are available here.
What are the acceptable effective ways to mark mice?
The IACUC regulates the marking of mice. Ear punching (Ear punchers: Fisher 01-337B, or Kent Scientific INS301202) can be done without anesthesia. The external ears are large enough to ear punch after 2 weeks of age. However, ear punches can become difficult to read after several weeks because of healing. For more permanent marking, removing the last joint of a toe without anesthesia during the first week after birth is acceptable. Only one toe can be clipped per limb. Anesthesia must be used for toe-clipping of mice older than one week. A scientific justification must be provided for the use of toe-clipping instead of other methods of identification. The complete CWRU IACUC policy on toe-clipping is here. Tattooing is an acceptable alternative, although it is less commonly used. India ink in a 1 ml syringe with a 30 gauge needle can be used to mark paws in different combinations. Alternatively, commercial tattoo inks and tattooing devices are available (http://www.ketchum.ca). In some instances, genotypes are needed at birth: tattooing with India ink of newborn paws with tail clipping works well in practice. Mice, including newborns, can be marked for a few hours with an indelible marker––however, the ink is quickly removed by the moms or by grooming making remarking necessary. Implanted ID transponder chips are an alternative, if cost and labor are no obstacle.
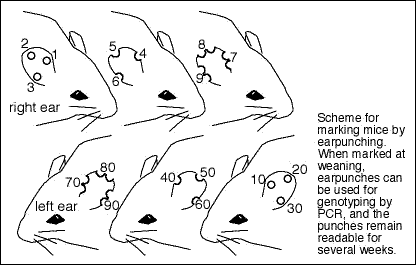
How are mice genotyped?
An efficient way to manage your mice is to wean, ear punch, and genotype at the same time. Genotyping by PCR is the most efficient. Ideally, the PCR primers are specific to the mutation, rather than a generic set like primers to neoR or lacZ. Extensive information on developing and validating assays for genotyping mice is available here. A simple and reliable protocol for PCR from ear punches is provided here. Alternatively, Southern blots can be performed on toe DNA prepared by the method outlined here.
How are males and females housed? Don't the males fight?
Females can be housed five to a cage, and can be mixed with unfamiliar females without problems. Special attention must be paid to the housing of males because of their propensity for fighting. Males will generally not fight if they are housed together from before sexual maturity through to old age. After sexual maturity, males will fight when introduced to a new male. For example, males that have been housed alone will fight with any introduced male. Therefore, stock males from the same litter should be housed together from a young age to conserve space. Males that are used as stud males are housed one per cage, and are never placed into a cage with other males. Signs of fighting between males manifest as bite wounds and can result in death. Females housed together will sometimes not get along and this can manifest as whiskers trimmed to the root, other sharply demarcated areas of hair loss without skin lesions (barbering), or bites on the back and hindquarters. Most often this behavior can be eliminated by housing the females in question at a lower density or by removing the dominant female (the one that still has her whiskers and has no bites). Mild to moderate barbering may not require separation, but merits closer observation in case aggression escalates.
What is a plug?
Plugs are useful for obtaining timed matings. A plug is hardened semen blocking the vagina, and remain in place for about 12 hours after mating. Plugs are detected by visual inspection or by probing gently with a sterile toothpick or blunt probe (Fisher seeker 08-995) on a female immobilized as described above. Mating is assumed to occur at the midpoint of the dark cycle (midnight under a 12 hour on/off cycle starting at 6), and thus noon of the next day is 0.5 days of gestation. For a complete description of the stages of mouse embryogenesis and fetal development, see Hogan, B. L. M., Beddington, R., Costantini, F. and Lacy, E. (1994). "Manipulating the Mouse Embryo. A Laboratory Manual." Cold Spring Harbor Press.
How do I tell if a mouse is in estrus?
Female mice in estrus will be receptive to mating. By picking females in estrus, you can maximize the breeding of your mice, or obtain multiple females mated at the same time. You should expect two-thirds to three-quarters of mice in estrus to mate, on average. Females in estrus have swelling of the lip of the vulva closest to the anus. Pick up the female by the tail in the proximal third, and with thumb and index finger holding the tail, let the mouse grasp the cage bar with forepaws and gently press down with the other fingers on the lower back and sacrum to tilt the genital-anal region up (a lordotic position). In estrus, the vulva is swollen, but the vagina does not gape open.
The estrus cycle is 4 to 6 days, so about 1 in 5 females on average should be in estrus at any time if the females are cycling randomly. However, females housed continuously together can cycle together, or can exit the estrus cycle. Young females (6 to 8 weeks) are less likely to have stopped cycling. Exposure to male pheromones will restart the cycle, as can changing social groupings among females. Transfer of bedding from a sexually mature male's cage can be used to stimulate cycling.
My mice are not breeding. What can be done to promote reproduction?
Mice breed best if they are less than eight months old, so keep track of the age of your mice. Strains with reduced fertility breed best when they are young, but even the most robust strains don't breed well after they are a year old. Know what to expect from your strain: the Jax listing of strain characteristics is useful in this regard. The amount of fat in the diet can have a significant effect on female fecundity (more fat, more fecund), but increased fat can have a detrimental effect on stud performance. The ARC can provide your mice with an alternate chow with a higher fat content (standard diet is Purina 5010, a low fat chow; Purina 5021 is a high fat chow). Sudden noises can have a detrimental effect on breeding, as can poor air quality. Privacy provided by "love shacks" (K.L.A.S.S. 4960 Almaden Expressway, Suite 233, San Jose, CA 95118, USA. Tel: (408) 266-1235 mouse nesting boxes MB-01) or nestlets (VWR 10279-140) can help shy strains. The light-dark cycle has significant effects on mouse reproduction. Ensure that your mice are on the appropriate cycle (12 hours light, 12 hours dark). In some cases, extending the light period (14 hours light and 10 hours dark) can improve reproductive success.
My mouse has closed or enlarged eyes, tumors, alopecia, or seizures: what is wrong?
Mice can suffer from a variety of illnesses. The spectrum of diseases is dependent on strain, housing conditions and a wide variety of other conditions, but the short list above gives many of the common ailments of mice. However, discuss the health of your mice with the veterinarian often. The ARC Morbidity and Mortality Report (MMR) card may be used by research staff to label the cage of an ill mouse to obtain examination of the animal by an ARC veterinarian. Place the back hard copy with the ARC logo on the animal's cage and deliver the top two copies to the veterinary technician office, EB12A.
Useful resources on mouse health include
The Jackson Laboratory’s list of inbred strains of mice, which gives the susceptibility of different strains of mice to disease.
Guidelines for assessing the condition and health of mice, a pdf file of a paper from Lab Animal.
The Comparative Pathology web site at UC Davis
The American Committee on Laboratory Animal Disease (ACLAD) web site.
Should I be concerned about the genetic background of my mutant mice?
Genetic background can have a significant effect on mutant phenotype. For many mutants, you will want to have your mutation on a well characterized, robust, common inbred strain like C57Bl/6. A mutation typically is crossed onto the C57Bl/6 background for 10 generations, at which point it is considered congenic since the genome is expected to be 99.8% C57Bl/6. (Detailed information on the expected content of the genome at each backcross generation is available in Lee Silver's book, Mouse Genetics available online at The Jackson Laboratory). The mutation can be continued to be crossed to the inbred strain after this point. Mutants maintained by breeding among themselves can result in the fixation of new mutations within the strain, and so should be avoided. Throughout the breeding, knockout or transgenic strains genotyped by PCR should occasionally be verified by Southern blotting, since the vagaries of PCR have caused more than one lab to lose a mutant. Transgenic strains often lose expression of the transgene irreversibly through methylation of the insertion site, so it is wise to check transgenic lines for expression at each generation. Cryopreserve your strain, if it is not one of the common, commercially available strains. Cryopreservation is available from local (Case Transgenic and Targeting Facility and commercial services (Jax; Charles River). In some instances, the greater robustness and reproduction of an outbred strain like CD1 (from Charles River) is a sufficient advantage to offset the heterogeneity of the background, say, for example, in studies of embryogenesis.
A number of the strains commonly used for making transgenic mice either are blind (FVB/NJ), or segregate a gene for blindness (B6SJLF1/J and B6CBAF1/J). The blindness in these strains is caused by recessive retinal degeneration by weaning due to the Pde6brd1 mutation. A listing of the affected strains, and a discussion of how to cope with this problem is here.
Many inbred strains (including C57BL/6J) have the age-related hearing loss 1 (Ahl1) mutation, which causes degeneration of hearing starting at about 10 months of age, depending on genetic background (Johnson et al., (2000) Genomics 70:171).
How large a colony of mice should I maintain?
The size of your mouse colony depends upon your needs. Given the costs of keeping mice, you should keep your colony as small as is practical. For strains that you are not currently using, a small breeding colony of several cages is sufficient (letting it get down to one cage is living on the edge--don't do it). Mice that don't breed are a dead end, so make sure that if you have reduced your holdings of a strain to a minimum, that the mice are fertile and young. Schedule breeding of replacement breeders so that breeders may be replaced when they are 6 to 8 months of age. Cryopreservation of mutants and strains is highly recommended for insurance against accidental loss. The structure of a breeding colony will depend on your needs. For those who need timed matings, a set of stud males individually housed and cages of non-pregnant females housed five to a cage are essential. For maintaining stocks by breeding, breeding pairs (male and female and litter) are typically part of the colony as well. A nice discussion of efficient breeding strategies to meet your needs is given by the UC Irvine Transgenic Core.
How extensive should my breeding records be? What should I track?
Your particular needs will determine the level of detail that you will need in your breeding records. With large colonies, detailed record keeping can consume significant resources. However, detailed records are essential to solving problems when they arise. Records can be kept in a combination of lab notebooks and cage cards, in user-built databases, in commercial purpose-built databases (Bigbench Mouse, Progeny's pedigree software) or in free databases (Laboratory Animal Management System (LAMS)) or in a FileMaker database, using templates provided by others Caleb Davis' MouSeek, various FileMaker Database Templates constructed by different labs.)
How do I ship/receive mice?
All mice to be received at CWRU from institutions other than approved commercial vendors must be approved for receipt by the ARC. The Nonstandard Vendor Form (which can be downloaded as a pdf file here must be completed, a health report on the mice must be submitted, and a CWRU ARC veterinarian must approve shipment. Shipment must be directed to the receiving department at the Health Sciences Animal Facility. Once you are approved for shipment by the veterinarian, they will give you the address for receiving. Under no circumstances are mice to be received without prior approval. Depending on the pathogen status of the mice, they may be approved for receipt into the clean or dirty quarantine. A major source of mouse pathogens is mice received from investigators at other institutions. The methods used for infectious agent monitoring are relatively insensitive, and exposure of mice to pathogens can occur during shipping. The best way to ensure that pathogens are not introduced is to rederive the incoming strain. The health status of all incoming mice not from commercial vendors are reviewed by the ARC, and they will make the decision whether rederivation or treatment is required before release from quarantine. Rederivation of mice can be performed by the Case Transgenic and Targeting Facility. The most expeditious way to ship and rederive is to ship frozen embryos or sperm, or live preimplantation embryos. Preimplantation embryos can be shipped, either cryopreserved in liquid nitrogen, or as blastocysts at room temperature by overnight delivery. Contact a transgenic service about cryopreservation and shipping frozen or room temperature embryos. Alternatively, live mice can be shipped. Shipping containers for mice can be purchased from Taconic and Zivic Miller. Food and water can be provided with "Napa Nectar" packets, available from Lenderking. You should be aware that shipment of females in the first third of pregnancy usually results in the resorption of the embryos. Keep the weather forecast in mind when shipping: you don't want to ship in extreme cold or heat. When you are shipping mice to other institutions, they will want to know the health status of your mice and will probably want to contact the ARC veterinary staff.
The ARC can assist with shipping mice to other institutions (Nonstandard Vendor Export form). (Some institutions will accept health info only from the animal facility, not from the sending PI.)
How do I keep mice free from pathogens?
There are a number of different levels of pathogen control. These levels run from commercial operations providing for axenic (sterile) and gnotobiotic (defined flora) housing, to the athymic and ultrabarrier facilities at CWRU, the ventilated rack systems of the Health Sciences Animal Facility and the Wolstein Mouse Facility, Static Microisolators to conventional caging. The majority of mice at CWRU are Specific Pathogen-Free. The procedures that you need to follow are set by the ARC and will depend upon the type of housing. However, a few general rules may be observed in all instances. You should be consider that mouse pathogens will be most prevalent in mice, and therefore contact with mice which are potential pathogen carriers should be avoided: no pet rodents at home; escaped and wild mice in colonies should be trapped immediately; avoid contact with mice known to carry pathogens.
UC Irvine University Transgenic Core has an excellent guide to mouse breeding and reproduction
University of Michigan Transgenic Core guide to breeding transgenic and knockout mice
The Jackson Laboratory has a Mouse Breeding Strategies Manual (.pdf)
The Mouse as a Model System, information compiled by us about the genetics and biology of the mouse.
The CWRU ARC provides hands on training in microisolator technique and mouse handling.
Useful information on the background and practical side of mouse genetics can be found in Lee Silver's Mouse Genetics, now published online by The Jackson Laboratory.
Hogan, B. L. M., Beddington, R., Costantini, F. and Lacy, E. (1994). "Manipulating the Mouse Embryo. A Laboratory Manual." Cold Spring Harbor Press
Hetherington, M., Doe, B. and Hay, D. (2000). Mouse care and husbandry. In: "Mouse Genetics and Transgenics: A Practical Approach." Jackson, I. J. and Abbott, C. M. editors. Oxford University Press.