What You Will Provide Part I
- An account number.
- A mycoplasma-free ES cell line with 40 chromosomes.
- We will inject approximately 50 blastocysts per ES cell line. This should result in 10 mice born. Three of the mice are expected to be strong male chimeras. We have a proven record of success.
- We will house the mice until weaning (three weeks after birth).
- You will house the weaned mice.
- $4,000 per 129 cell line and the cost of donor females; $5,500 per cell line and the cost of donor females for other genetic backgrounds.
- Local, non-CWRU orders will be charged an additional $300 for packing and transporting mice. Non-local orders will be charged $300 and the costs of transportation.
- Investigators need an IACUC protocol from their institution to receive mice from the core. CWRU IACUC protocols need to specify use of the Case Transgenic Core. Investigators do not need an IBC protocol of their own, normally: the review for recombinant DNA in animals will be conducted on the information submitted through online ordering for the transgenic core, and will be added to the core's IBC protocol as an amendment if approved. You may be contacted by the IBC for additional information as part of rDNA review after submission of the order. IBC approval is required prior to initiation of injections.
- We ask that you acknowledge the Case Transgenic and Targeting Facility in seminars and publications.
- From the injection date of cells, it will be a minimum of 6 weeks before we will be able to deliver mice to you (almost 3 weeks for gestation, and at least 3 weeks of postnatal growth before weaning). It will be another 6 weeks before the male chimeras wil be old enough to breed, and a minimum of another 6 weeks before you will have weaned offspring to genotype to test for germ line transmission--a total of 4.5 months from injection to testing for germ line transmission.
- The current (3/12/26) expected time between order placement and ES cell injection is 8 weeks. During the 8 weeks prior to injection, the chimera request is reviewed by the rDNA committee of the IBC, the ES cells are thawed and grown, and mice are ordered and received.
Online Ordering
- PI name, address and contact info
- Account Number
- IACUC protocol number
- Cell line name
- Parental cell line name
- Genetic background of the cell line
- Order ID for a CWRU knockout, or the repository the cell line was obtained from
- Purpose and design of the animal experiments
- Name, and function of the gene
- Whether the gene is involved in infectious disease in normal healthy adult humans (yes/no)
- Whether the gene presents any hazard to health or the environment (yes/no)
- A .pdf file of a map of the targeting strategy
Success Rate in Generating Chimeric Mice
The core generates an average of about 5 chimeras per cell line.Getting Started
The likelihood of germline transmission of a targeted mutation is increased with the number of independent ES cell lines available. On average, about two-thirds to three-quarters of ES cell lines on the 129 genetic background will go germline. For ES cell lines on the C57 background, an average of only one-half to two-thirds of lines will go germline. Therefore, if you are purchasing targeted ES cell lines from a public repository, we recommend that you purchase 3 or more correctly targeted lines on the 129 background, and 4 or more lines on the C57 background. Likewise, order 2 or more injections for 129 lines, and 3 or more for C57 lines. For the generation of tissue-specific knockouts with conditional alleles in ES cells from EUCOMM, see our primer.
Frequently Asked Questions
What are chimeras?Typically, chimeras are constructed to generate mice from gene-targeted or gene trapped ES cell lines. ES cells injected into host embryos give rise to mosaic mice known as chimeras.
Male ES cells are injected into unsexed blastocysts. If the host embryo is female and the male ES cells make germ cells, the chimera will often be a fertile male. Host embryos from a strain which won't compete too strongly with the ES cells are used, and the two components (ES and host embryo) are genetically marked with coat color genes so that the contribution of the ES cells to the animal can be determined easily. If the proportion of ES cell descendents in the coat of the animal is high, the probability that ES cells are represented in gametes is also high, since ES cells mix thoroughly with host cells early in embryogenesis. The coat color marking system also makes it possible to determine, with appropriate crosses, if the offspring of the chimeras are from the ES cell component or host embryo component.
129 ES cells give rise to brown coat color because they are A/A (wild type Agouti), and the C57BL/6J host embryos give rise to black coat color because they are a/a (recessive nonagouti). The ES cells are from the 129 strain of mice; the host embryos are from the C57BL/6J strain of mice. If these chimeras are bred to a/a non-agouti mice (for example C57BL/6J, then any brown offspring (A/a) must have arisen from ES cell-derived gametes, and 50% of the brown offspring are expected to carry the knockout allele. Alternatively, the transmission of the mutation can be assayed directly by PCR or Southern blot assays of tissues from the offspring.
If the ES cells are from the C57BL/6J strain (a/a, Tyr/Tyr and black), the host embryo will be albino C57BL/6J (a/a, Tyrc-2J/Tyrc2-J and white). Breeding such chimeras to albino C57BL/6J can generate black offspring (a/a, Tyr/Tyrc-2J) from the ES cell component, 50% of which should carry the mutation, and white offspring (a/a, Tyrc-2J/Tyrc-2J) from the host embryo component.
The male chimeras which are likely to transmit the targeted mutation can be identified by copulatory plug genotyping.
Chimeras for other uses
We also make aggregation chimeras and diploid<==>tetraploid chimeras. Two preimplantation embryos from two different strains of mice are combined to form an aggregation chimera. This technology can be used to generate genetic mosaic embryos for analysis of autonomy and nonautonomy of gene action and other experimental goals. In diploid<==>tetraploid chimeras, the tetraploid component largely gives rise to much of the embryo-derived placenta and the diploid component to the embryo. This type of chimera can be used to determine if a gene is required in placenta or embryo.
Why are my mice funny colors?
Some ES cell lines are heterozygous for coat color alleles that only reveal themselves in subsequent generations. The R1 129 ES cells we use are heterozygous at albino locus, carrying one copy of chinchilla allele of albino (cch, also designated Tyrc-ch) and are heterozygous at the pink-eyed dilution locus (p). Breeding offspring descended from R1 ES cells to each other can give rise to mice which are black, different shades of gray, different shades of brown, or yellow, depending on the assortment of coat color alleles.
What can be done to ensure germline transmission?
Germline transmission is maximized with ES cells
that have spent a minimum amount of time in culture, that have a normal complement of chromosomes, and that are free of yeast, bacteria and mycoplasma. Target a cell line that you know is transmitted under your conditions at your institution. Use a parental cell line that is at the lowest passage number available (preferably less than passage number 16). Minimize the time that the targeted line is cultured. Verify that targeted cell lines have the normal number
of chromosomes. Test your cell line for mycoplasma. Some cell lines will not transmit even when all these parameters are in your favor. Even under optimal conditions, only two-thirds of targeted ES cell lines will be transmitted through the germ line. Therefore, start with at least three independent targeted cell lines to ensure transmission.
I have weak chimeras/female chimeras. Will they transmit the knockout?
Both weak chimeras and female chimeras can transmit mutant alleles, although infrequently. Male chimeras which are likely to transmit the targeted mutation can be identified by copulatory plug genotyping. Only about 10% of female chimeras transmit the ES cell genome, when they do so it is only in the first few litters, and many of their male offspring have sex chromosome aneuploidies which render them infertile (Bronson et al., 1995 PNAS 92, 3120 [.pdf]).
Why did my strongest chimeras die?
Mortality can increase with the proportion of the animal that comes from ES cells, even for the best cell lines. This mortality is due largely to epigenetic aberrations which arose in culture. The epigenetic aberrations are thought to be corrected by subsequent breeding of surviving mice, and would segregate randomly away from the targeted locus, even if they were not.
What strain of mice do I breed my mutants to?
To minimize genetic variability as quickly as possible breed the chimeras to the same background as the ES cells. If the ES cells were 129, breed the chimeras to 129 mice and your mutation will be completely on the 129 inbred background in one step. The most common inbred background for genetic studies is the C57BL/6 strain. If your ES cells are C57BL/6, cross your chimeras to C57BL/6J mice. If you wish to change the background, the standard is to do 9 serial crosses to the inbred strain of choice (about 2.5 years). The rationale for the 9 generations is explained here by Lee Silver. Alternatively, about half the number of generations is required by marker-assisted backcross, or speed congenics.
For maximum reproduction, cross your mice onto an outbred strain like CD1. CD1 mice were derived from Swiss mice, have a high degree of genetic variation and were selected for robust reproduction.
ES Cells with Conditional Alleles from EUCOMM
Each cell line from EUCOMM will require you to complete an Materials Transfer Agreement. Start this process as early as possible.
The generation time of mice and the number of crosses necessary to generate the experimental mice combine to a length of time which is surprising to those not used to mouse genetics, so plan ahead. The generation time of mice (the time from sexually mature adult to sexually mature offspring) is about 3 months.
The EUCOMM conditional alleles typically have a Frt-flanked cassette (a "Frt-ed" cassette). The cassette has a splice acceptor which diverts virtually all splicing into the cassette, making the allele a loss-of-function mutation in this configuration. To generate the conditional allele, the Frt-flanked cassette needs to be removed by crossing to Flpe-expressing mice. The Flpe/Frt system is comparable but distinct from the Cre/LoxP system.
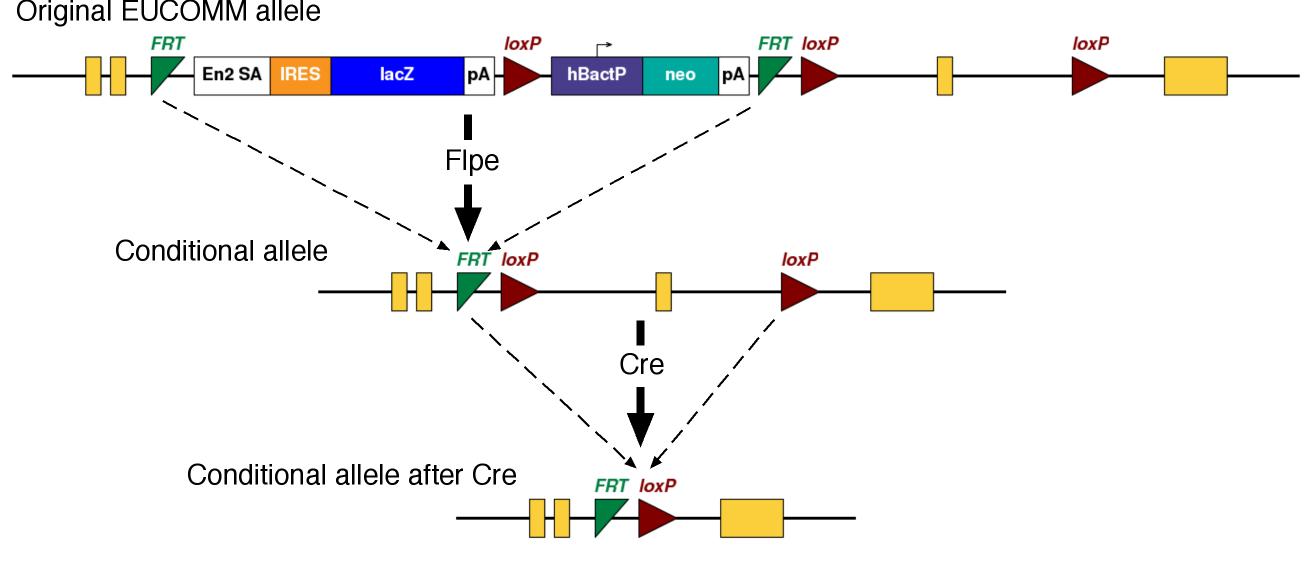 We will inject the ES cells into host embryos to make chimeric mice. From there, you will breed the mice to generate mice carrying the mutation (germline transmission). This founder generation of mice can be crossed to mice which express Flpe to remove the Frt-ed cassette, generating the conditional allele. Then, you need to do several more crosses to generate the experimental mice.
We will inject the ES cells into host embryos to make chimeric mice. From there, you will breed the mice to generate mice carrying the mutation (germline transmission). This founder generation of mice can be crossed to mice which express Flpe to remove the Frt-ed cassette, generating the conditional allele. Then, you need to do several more crosses to generate the experimental mice.
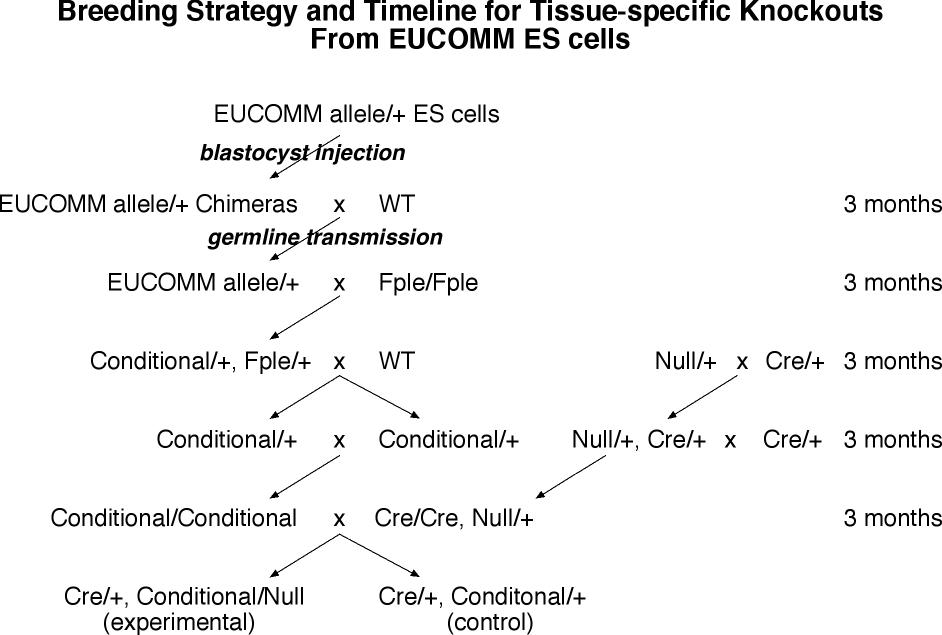
- From ES cell injection to adult sexually mature chimeras is the generation time of mice (3 months).
- The chimeras are bred to wild type mice to establish the mutant in the germline (3 months).
- The heterozygous frtd conditional mice are crossed to Flpe expressing homozygotes. This generates Flpe/o, frtd conditional/+ offspring. (3 months)
- The Flpe/o, frtd conditional/+ mice are crossed to wild type to establish the excised allele in the germline and breed away Flpe, and excision is verified molecularly in these conditional/+ mice (3 months).
- The conditional/+ mice then bred to each other to make homozygous conditional allele mice (3 months).
- The conditional/conditional mice are bred to tissue-specific-Cre/tissue-specific-Cre, null/+ mice to make the experimerntal tissue-specific-Cre/o, conditional/null mice, and control tissue-specific-Cre/o, conditional/+ mice.
Also, the conditional allele should be analyzed to determine if it is wild type in function before excision with Cre, and null after excision with Cre. To determine if the conditional allele is wild type in function before excision, conditional/conditional and conditional/null mutants should be examined to determine if their phenotype is the same as the +/+ and null/+ mice respectively. To determine if the Cre-excised conditional is a null allele the conditional is crossed to a germline Cre mouse and the excised conditionals are intercrossed to test if the phenotype of excised conditional/excised conditional is the same as null/null and/or that no protein is made from the excised conditional allele.
A reporter of Cre activity like RosaReporter, or more ideally, showing directly that the protein of interest is absent from the target cells by immunofluorescence is an important control. RosaReporter generates permanent beta-galactosidase expression in all cells which were exposed to Cre.
Excessive Cre expression can lead to chromosome breakage which can cause phenotypes unrelated to the function of the conditional allele. Therefore, sibling mice which express Cre but retain some gene function (for example, conditional/+) are an important control.